CDC advisory panel unanimously approves expanded COVID vaccine boosters
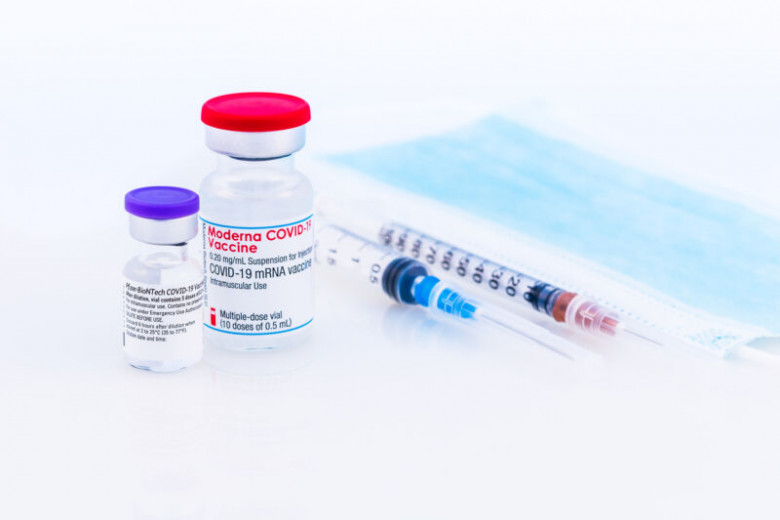
On Thursday, the Centers for Disease Control's expert advisory committee on vaccines met to vote on new guidelines for the use of boosters to sustain the immunity provided by the COVID-19 vaccines in use in the US. The day prior, the Food and Drug Administration issued an emergency use authorization (EUA) that greatly expanded the number of vaccinated people who could receive a booster shot. That set the stage for the CDC to determine whether the FDA approval should be adopted as formal health policy.
A key step in the CDC's policymaking process is approval by its Advisory Committee on Immunization Practices (ACIP). While the CDC director isn't bound to follow ACIP's advice (and notably didn't in an earlier booster decision), overruling ACIP is unusual. Given that ACIP has now voted unanimously to expand booster use to Moderna and Johnson & Johnson vaccine recipients, the CDC director will likely follow its guidance.